- +Products
- +Allergen testing
- +Culture Media - reagents and reference material
- +Environmental testing solutions
- +Laboratory Consumables
- +Laboratory equipment
- +Microbiology equipment
- +Neogen® Food Safety Solutions
- +Temperature & Humidity Monitoring
- Services
- Support
- News
- +About Us
- Contact
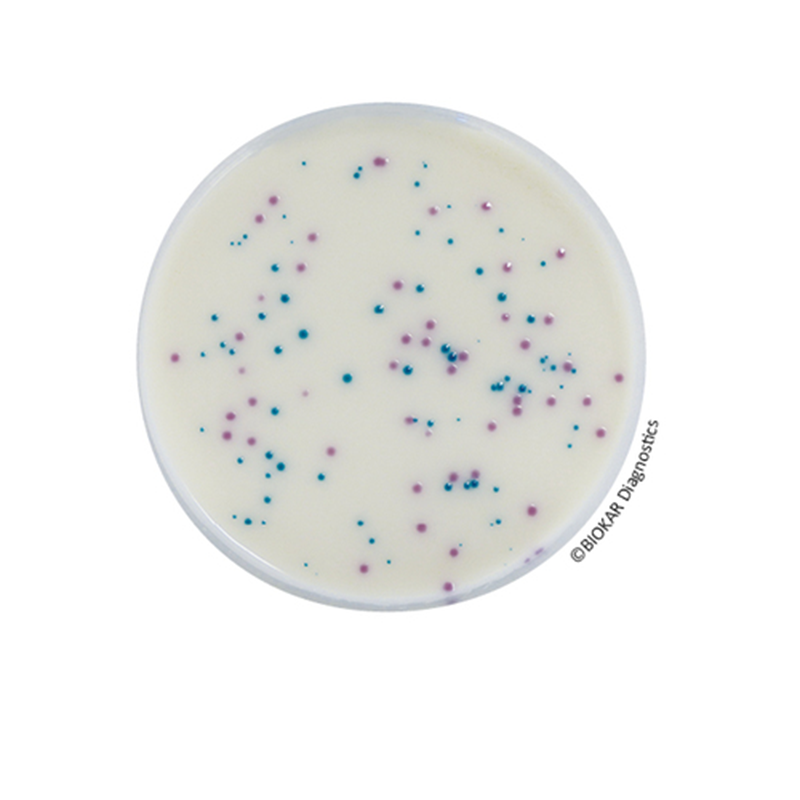
IRIS Salmonella
IRIS Salmonella® is an alternative research method of Salmonellae in human food and feeds, and environmental sample (except primary production samples).
Studies performed on IRIS Salmonella® Agar show a high specificity for the detection of Salmonellae including atypical species and serovars, which is a source of confusion on other medium.
Indeed, the detection of Salmonella Typhi and Paratyphi, lactose-positive Salmonellae (Salmonella Senftenberg and subspecies S. arizonae and S. diarizonae), saccharose-positive strains are ensured. The medium allows the detection of non-motile serovars (S. Pullorum and S. Gallinarum) or monophasic strains. IRIS Salmonella® Agar allows also the detection of strains which show a light or absence of esterasic activity on other medium (Salmonella bongori, Salmonella Dublin and Atento, certain strains of S. enterica, S. houtenae and S. diarizonae subspecies).
Available in formats of 20 and 120 ready to use plates
In compliance with regulatory requirements, a USDA permit (VS 16-3) is mandatory for shipments from Canada to the USA due to the inclusion of peptone, an animal byproduct.
The method is NF VALIDATION certified, with the IRIS Salmonella® supplement, according to the NF EN ISO 16140- 2 validation protocol of 2016 for the following categories:
- All food products (from 0 to 25 g)
- All animal feed and pet food (from 0 to 25 g), dry animal feed and pet food (from 50 g to 125 g)
- All samples from the industrial production environment
- Milk powders (including infant milk powders with and without probiotics) from 50 to 375 g
The method is also certified NF VALIDATION with the CSD supplement according to the validation protocol NF EN ISO 16140-2 of 2016 for the following categories:
- Infant milk powders, with and without probiotics; ingredients for test intakes up to 50 g, with a 1/10th dilution
- Infant milk powders, with and without probiotics; ingredients for test intakes of 50-375 g, at 1/4 dilution
- Samples from the production environment
Refer to the certificate available on the NF VALIDATION website for the end date of validity of the method. The reference method used for the validation is the standard NF EN ISO 6579-1 of 2017. IRIS Salmonella® Agar may be used in the standard methods for the detection of Salmonellae as second isolation medium. The CSD supplement allows the common enrichment of Salmonella and Cronobacter in infant milk powders with and without probiotics, ingredients, and environmental products. Refer to the CSD method, BKR 23/12-12/20, certified NF VALIDATION, for the detection of Cronobacter spp.
The method allows the detection of motile and non-motile Salmonellae. Analysis may be declared negative after 37 hours of enrichment (Salmonella Enrichment) and differentiation (IRIS Salmonella® Agar) steps. The 1/10 dilution step of the sample is performed in Salmonella Enrichment broth according to NF EN ISO 6579 recommendations. The enrichment step is performed by adding the IRIS Salmonella® selective supplement to the broth previously mixed with the sample to be analyzed. After this addition, the enrichment broth turns green.
The obtained Salmonella Enrichment broth is incubated for 16 to 24 hours at 41.5 ± 1.0 °C for the general. The differentiation step is performed by re-streaking the broth on IRIS Salmonella® Agar and incubating for 21 hours at 37 ° C. Salmonella colonies are magenta. The selective agents permit the inhibition of Gram-positive and some Gram-negative bacteria. The secondary flora presents blue, purplish or colourless colonies. An eventual confirmation step may be done by classical tests described in standard methods or by a Latex test directly from an isolated magenta colony from IRIS Salmonella® Agar



