- +Products
- +Allergen testing
- +Culture Media - reagents and reference material
- +Environmental testing solutions
- +Laboratory Consumables
- +Laboratory equipment
- +Microbiology equipment
- +Neogen® Food Safety Solutions
- +Temperature & Humidity Monitoring
- Services
- SUPPORT
- News
- +About Us
- Contact
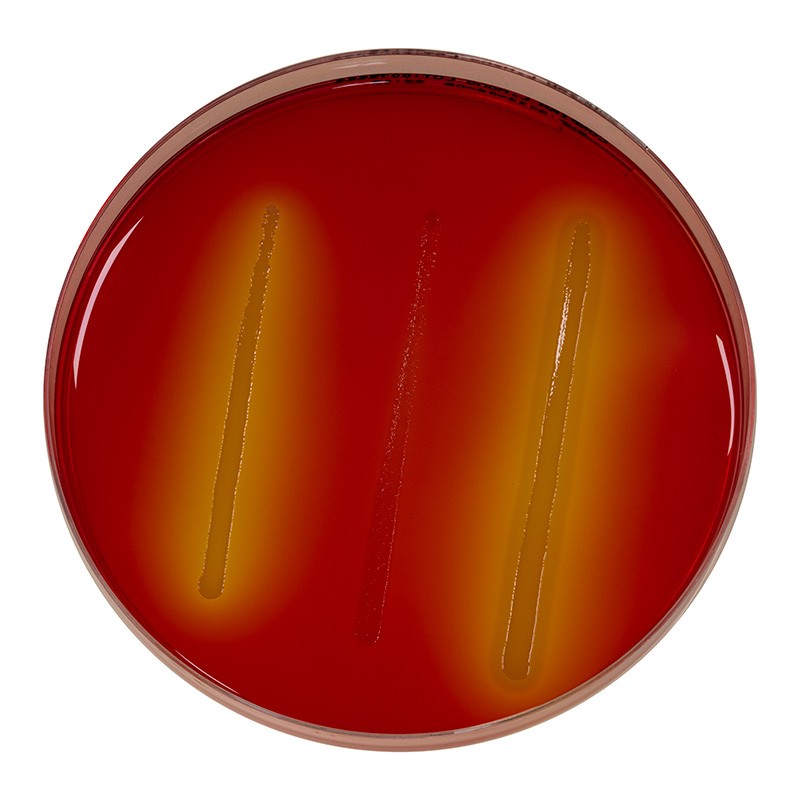
Confirm’ L. Mono Agar®
CONFIRM’ L. mono Agar ® is a solid culture media designed for the confirmation of the genus & species Listeria monocytogenes, isolated from a single characteristic colony from COMPASS ® Listeria Agar, within the context of the detection method (certified AFNOR Certification, under the reference number BKR 23/2-11/02 and enumeration methods (certified AFAQ AFNOR Certification under the reference number BKR 23/05-12/07).
Available as ready-to-use medium: 10 Petri plates Ø 90 mm.
In compliance with regulatory requirements, a USDA permit (VS 16-3) is mandatory for shipments from Canada to the USA due to the inclusion of peptone, an animal byproduct.
The unique composition of the media in peptones and growth factors insure proliferation of strains belonging to the genus Listeria, isolated from colonies taken from COMPASS® Listeria Agar (refer to the technical data sheet for this product).
A judiciously developed inhibition system provides the selectivity needed to prevent proliferation of secondary microflora, which could be isolated along with the characteristic colonies from COMPASS® Listeria Agar.
The detection of rhamnose fermentation is visualized by a color change to yellow, the result of a localized drop in pH.
PI-PLC detection is observed through the development of an opaque halo around the inoculation streak.
Related Products